Salt in the preparation of the stock solution sce prelaboratory assigament question 2a.
Experiment 35report sheet spectrophotometric metal ion analysis.
Prepare a stock solution.
Prepare a stock solution.
Calibration of the spectrophotometer to set the lambda max.
Experimental procedure part a.
Experiment 35 report sheer spectrophotometric metal ion analysis j j date lab sec.
Introduction this experiment involves the use of absorption spectrophotometry to quantify concentrations of three metal ions co ii cu ii and ni ii based on differences in the absorption spectra of edta complexes of the ions.
Lab sec name desk no see prelaboratory assignment question 2a.
0 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 9 1 0 0 0 0 01 0 01 0 01 0 01 0 01 0 02 0 02 f x 0 02x 0 r 1 calibration curve of absorbance versus molar concentration for the standard.
This is a group experiment.
Osariemen emokpae professor adewale principles of chemistry lab i may 8 th 2018 lab report experiment 35.
Consider a sample of some solution contained in a small transparent vessel perhaps a test tube.
The purpose of performing this laboratory experiment was to learn the adaptability of spectrophotometric analyses to use the graphing techniques for data analysis and use a spectrophotometer to measure the concentration of a metal ion.
Experiment 35 spectrophotometric metal ion analysis lab report laboratory questions 3 4 5 6 3.
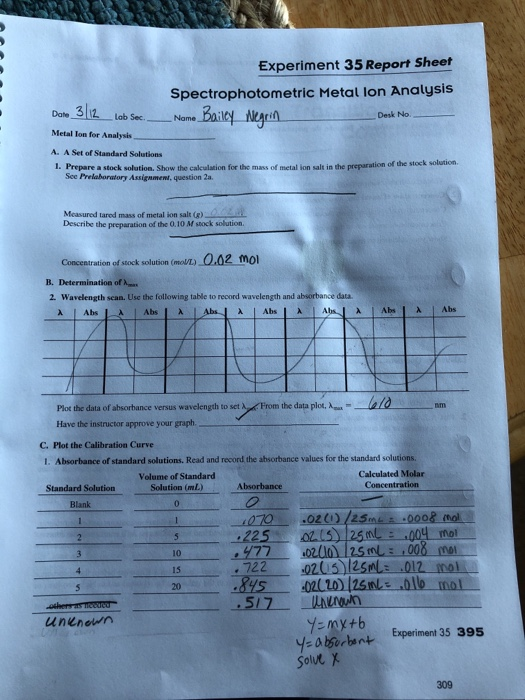
Experiment 35 report sheet spectrophotometric metal lon analysis date metal ion for analysis a.
Experiment 35 report sheet spectrophotometric metal ion analysis date 3 12 lab sec name metal ion for analysis a.
Spectrophotometric metal ion analysis introduction in experiment 35 the purpose was to use to a spectrophotometer to measure the concentration of a metal ion while using graphing techniques to analysis data.
View lab report experiment 35 report sheet from chem 100 at silver creek high school.
A set of standard solutions 1.
Total volume will be used many milliliters 2.
Experiment 35 boratory assignment spectrophotometric metal ion analysis lab sec.
Show the calculation for the mass of metal ion salt in the preparation of the stock solution.
Experiment the absorption of light of 522 nm wavelength by a sample solution will lead to an analysis for a trace amount of iron in an unknown sample.
Spectrophotometric metal ion analysis.
Solutions in part desk no.
A set of standard solutions 1.
We begin with a description of the spectrophotometric experiment.
All groups in each laboratory will share one set of standard solutions.