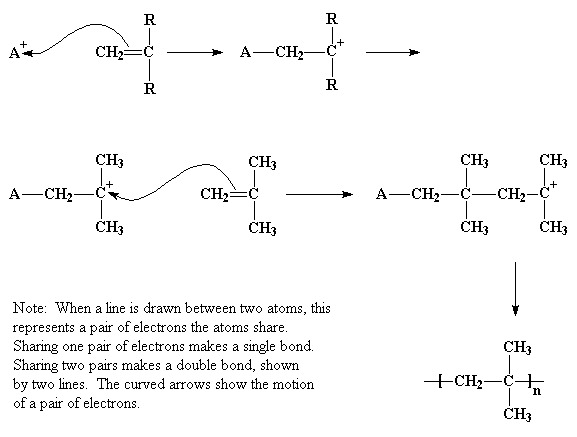Polymer with vinyl groups are usually polymerized to form hydrogel using redox reaction or through the use of thermal or photo initiator.
Free radical vinyl polymerization mechanism.
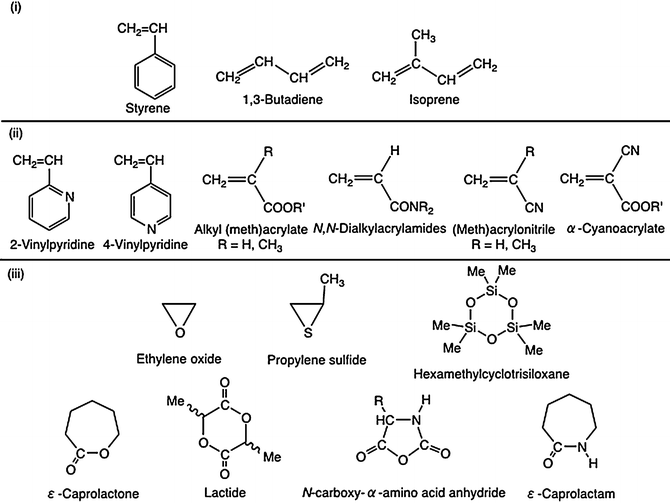
Virtually all of the monomers described above are subject to radical polymerization.
Covalent bond free radical.
Free radical polymerization frp is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks.
Free radicals can be formed by a number of different mechanisms usually involving separate initiator molecules.
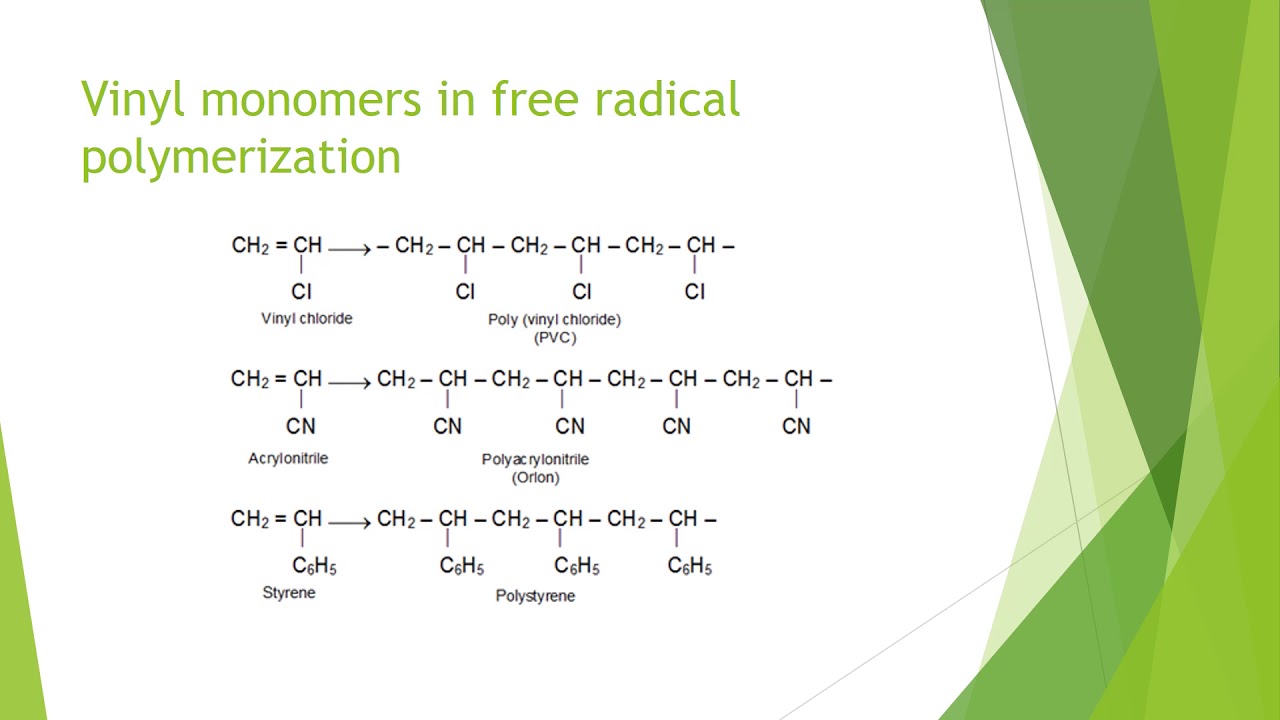
It is used to make polymers from vinyl monomers that is from small molecules containing carbon carbon double bonds.
Free radical polymerization is one of the most frequently used techniques in bioprinting to create chemically cross linked hydrogels.
Explains the process in polymer science known as free radical vinyl polymerization.
Deuterium isotope effect of cobalt porphyrin catalysed chain transfer in mma free radical polymerization was determined as the ratio of rate constants for reactions of perdeuterated or not deuterated monomer.
Since this can be initiated by traces of oxygen or other minor impurities pure samples of these compounds are often stabilized by small amounts of radical inhibitors to avoid unwanted reaction.
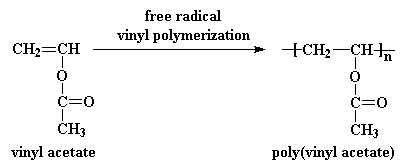
Into free radical polymerization of vinyl acrylate.
R m rm.
Keywords covalent bond free radical.
One of the most common and useful reactions for making polymers is free radical polymerization.
Photo induced polymerization can be broadly divided based on the initiation mechanism as radical cationic and anionic photopolymerization.
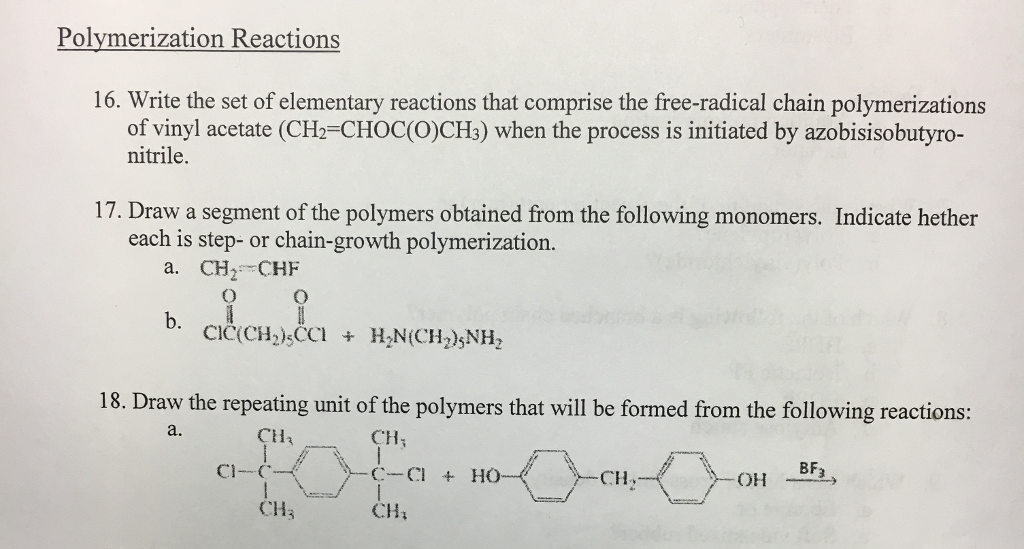
It is used to make polymers from vinyl monomers.
Explains the process in polymer science known as free radical vinyl polymerization.
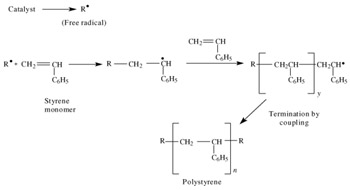
Mechanism of free radical polymerization.
Following its generation the free radical then reacts with a vinyl monomer that is it adds to one of the electrons of the double bond of the vinyl monomer and the remaining electron becomes the new free radical.
Free radical polymerization of vinyl monomers.
Following its generation the initiating free radical adds nonradical monomer units thereby growing the polymer chain.
Mechanism of polymerization of methyl methacrylate mma was studied by means of isotope effects.
99 natural abundance of 13 c kinetic isotope was applied to compare mechanisms of free.
6 3 2 1 free radical polymerization.
That is from small molecules containing carbon carbon double bonds.